Anti-wrinkle activity through skin profilometry
| Regulatory reference: | Proprietary method |
| Claim: | Anti-aging, Anti-wrinkle, Reduces signs of aging |
| Application field: | Raw materials and finished products. |
| Description of the test: | Blue laser skin profilometry: this technique allows you to carry out cosmetological studies of anti-wrinkle products with great reproducibility and accuracy thanks to a sophisticated instrumental and electronic set-up, which allows you to normalize the position of the volunteer and the test area in a millimetric way, an indispensable condition in conducting an anti-aging clinical study for the reduction of facial wrinkles. This clinical study allows a quantitative experimental evaluation of the anti-wrinkle effect through 2D measurements of wrinkles (Ra, Rz, LR, SPa, SPt, SPTm), and 3D (volume, average and maximum depth of wrinkles), and through quantitative morphological evaluations (mm2 of total surface covered by wrinkles). This allows the anti-wrinkle effect of any product to be assessed in the most appropriate way: immediate tensor effect, filler, firming effects (firming, toning, plumping, etc.) |
| Photo: | 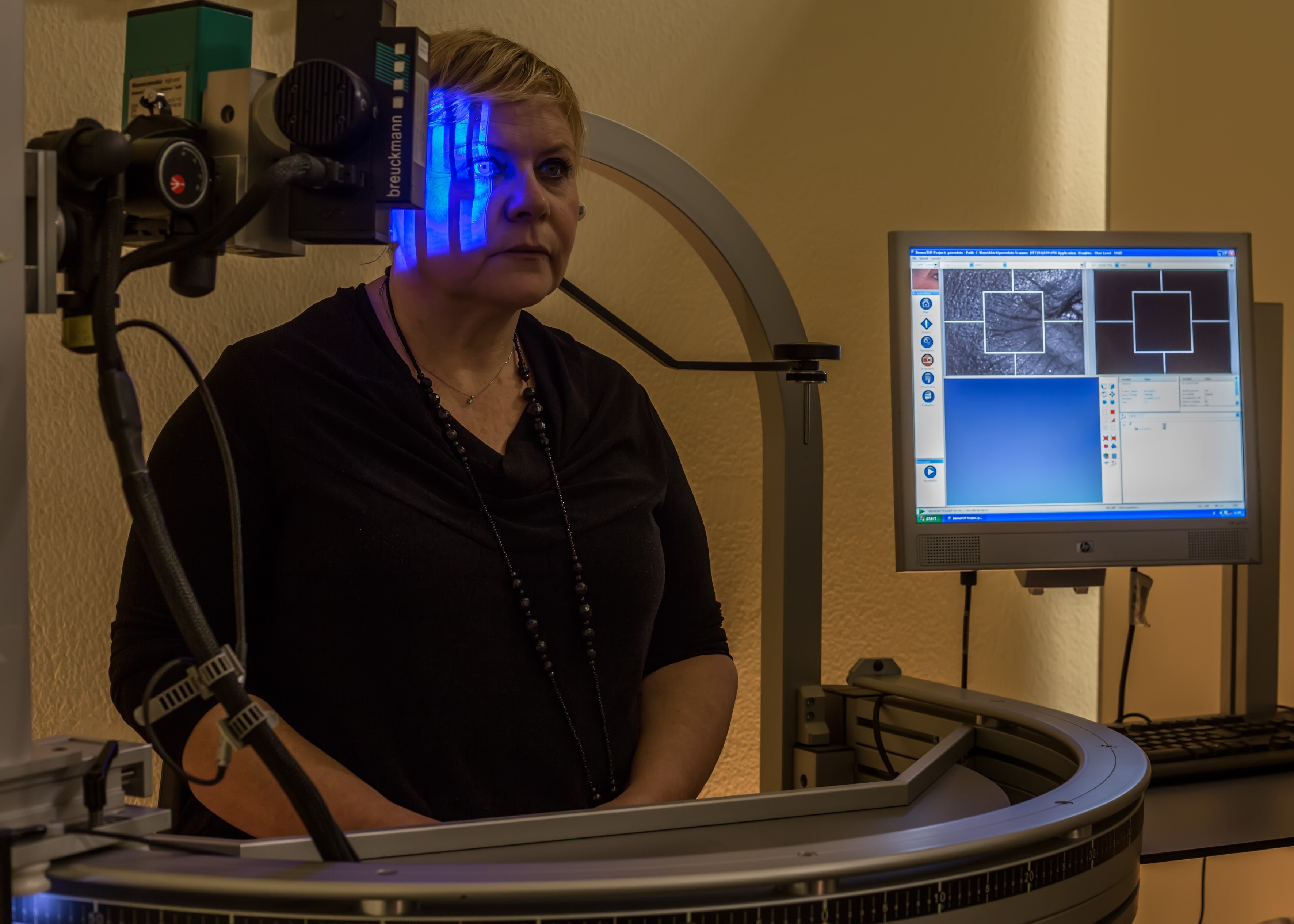 |
H295R Steroidogenesis Assay – OECD 456
| Regulatory reference: | According to OECD 456. |
| Claim: | No endocrin disruption |
| Application field: | Raw ingredients and finished products. |
| Description of the test: | The purpose of the test is identifying substances that interfere with 17-ß estradiol and testosterone production. |
Hyaluronic acid synthesis test
| Regulatory reference: | Proprietary method. |
| Claim: | Anti-aging and Reduces signs of aging |
| Application field: | Raw materials and finished products. |
| Description of the test: | In vitro evaluation of the synthesis of hyaluronic acid up to 48h following exposure to the sample, in several concentrations, on skin and dermal papillae fibroblasts. |
In vitro test for the evaluation of the protective effect against air pollution
| Regulatory reference: | Proprietary method |
| Claim: | Antioxidant and Anti-pollution |
| Application field: | Raw materials and finished products. |
| Description of the test: | In vitro test for the evaluation of the protective effect against air pollution. The purpose of this test is to evaluate the efficacy of the sample in reducing ROS release in response to a mix of heavy metals and atmospheric dust particulate in a skin-derived cell model. |
Cytotoxicity test for the identification of substances with acute oral toxicity LD50>2000 mg/kg
| Regulatory reference: | OECD 129: on using cytotoxicity tests to estimate starting doses for acute oral systemic toxicity tests; EURL-ECVAM 2011: follow-up study on the predictive capacity of the 3T3 Neutral Red Uptake cytotoxicity assay to correctly identify substances not classified for acute oral toxicity under the EU CLP system. |
| Claim: | Biocompatible and Non citotoxic |
| Application field: | Chemical substances or mixtures. |
| Description of the test: | The in vitro LD50 prediction test is designed to test chemicals or mixtures by evaluating their cytotoxic effect on an in vitro model of BALB/c 3T3 murine fibroblasts. |
Evaluation of the enzymatic inhibition of Tyrosinase
| Regulatory reference: | Proprietary method |
| Claim: | Depigmenting action |
| Application field: | Active ingredients, mixtures or finished products. |
| Description of the test: | Evaluation of the efficacy of a sample in inhibiting the activity of the tyrosinase enzyme in a controlled in vitro direct reaction. |
Evaluation of proliferation and protein synthesis on dermal papilla mesenchymal cells
| Regulatory reference: | Proprietary method |
| Claim: | Anti-hair loss and Strenghtening |
| Application field: | Active ingredients and finished products. |
| Description of the test: | Quantitative evaluation of the effects of a finished product or active ingredient on cell proliferation and protein synthesis through MTT test and total protein quantification after different periods of exposure. |
Ocular irritation test on reconstructed corneal epithelium
| Regulatory reference: | OECD n° 492 |
| Claim: | Non-irritant for the eyes |
| Application field: | Chemical substances or mixtures. |
| Description of the test: | Identification of chemical substances or mixtures that do not require classification as (severe) irritants through evaluation of their cytotoxic effects on an in vitro reconstructed corneal epithelium model. NOTE: if the sample qualifies as irritant, further tests are required in order to determine the degree of irritant action. |
Clinical simulation of use and ophthalmological evaluation on 20 healthy volunteers
| Regulatory reference: | Guidelines to Commission Regulation (EU) No 655/2013 |
| Claim: | Ophthalmologically tested |
| Application field: | Eye contour cosmetics or products that require to confirm the compatibility with the periocular mucosa in normal usage conditions. |
| Description of the test: | An ophthalmology specialist will inspect the target area, and especially the eyelids, before the application using a fissure lamp, in order to confirm the absence of swelling, hyperemia, dryness or desquamation. The inspection also includes any alterations of the eyelashes. In the eye itself, the integrity of the cornea and conjunctiva, the absence of hyperemia, and signs of conjunctival or tarsal irritation are verified. Finally, the absence of qualitative and quantitative alterations in the tear film. The specialist then evaluates and scores erythemal response, tearing, edema and dryness after the product has been used for 30 minutes, 2 days and 30 days to quantify possible alterations induced by the sample. The volunteers also provide subjective evaluations of itching, burning, stinging or foreign body response feeling, and/or sight clouding, at the same endpoints, using the VAS (Visual Analogical Scale) score system. |
Evaluation of hair restructuring efficacy
| Regulatory reference: | Guidelines to Commission Regulation (EU) No 655/2013 |
| Claim: | Anti-hair loss |
| Application field: | Hair products and cosmetics products for scalp treatment. |
| Description of the test: | In vivo test: performed on selected volunteers with specific trichological features (dry and damaged hair). Volunteers will apply the product according to pre-determined frequency and methods. 10 hairs from each subject will be gathered at T=0 and T= X days (duration to be defined with the customer). Dynamometric measurements will be performed in order to evaluate the increase in tensile strength of the hairs and their resistance to breakage. Subjective evaluations will be provided by volunteers using an open and multiple choices questionnaire to verify the perceived efficacy and other parameters such as effects on hair volume and agreeability. In vitro test: standardized locks of human hair (several types are available according to preferences) will be treated with a damaging mixture in controlled conditions. Part of the locks will then undergo treatment with the product. Comparative imaging will be performed under tangent light to highlight improvement of hair brightness. 2 samples will be taken from each lock to evaluate improvement by phase contrast microscopy or SEM. |